The Clinical Value of MPI
The Clinical Value of MPI
The Diagnostic and Prognostic Value of MPI
Considerations for the MPI Modalities and Your Patient
When a patient’s pretest probability of CAD is intermediate to high, myocardial perfusion imaging (MPI) may be an appropriate modality for risk stratification and patient management.1
Two MPI modalities are SPECT MPI and positron emission tomography (PET) MPI. Either can be used to identify ischemia and prior infarction, risk stratify patients with known or suspected CAD, and guide clinical management decisions regarding medical therapy or revascularization.2 There are some differences between these modalities that should be considered when selecting the right modality for the right patient.
PET has high diagnostic sensitivity and specificity, with high spatial resolution and attenuation correction, making PET a potentially superior cardiac testing modality for imaging patients who are more likely to have soft tissue attenuation artifacts.2 In addition, the radiation burden to the patient is reduced compared to other modalities.3 PET radiotracers also have a higher extraction fraction compared to the currently used SPECT radiotracers.4
-
PET has the potential to2:
- Quantify regional perfusion
- Calculate coronary blood flow reserve
- Measure the viability of myocardial tissue
-
The limitations of PET include:
- Lower availability and high cost of PET equipment3
- Short half-lives of PET radiotracers make exercise stress logistically challenging2
- The need for an onsite cyclotron or generator for the radiotracers used in imaging3
SPECT MPI continues to be the most commonly used imaging modality in nuclear cardiology today. It plays an important role in the risk assessment and evaluation of CAD and is widely available and accessible for patients who are known or suspected to have CAD.5
-
SPECT has the potential to2:
- Quantify regional perfusion
- Quantify coronary blood flow (with cadmium zinc telluride [CZT] SPECT cameras)
- Measure the viability of myocardial tissue
-
The limitations of SPECT include:
- Some perfusion images have limited quality4
- Global blood flow reduction may be difficult to recognize, and thus the extent of CAD could be underestimated5
- Contrast between normal and ischemic myocardium may be reduced5
Exercise Stress Testing
SPECT can be performed with exercise or pharmacologic stress. Exercise stress is the preferred imaging procedure because the ability or inability to adequately exercise has independent prognostic and diagnostic value.2 Exercise capacity is a strong indicator of long-term risk, including death, for men and women with known or suspected CAD.1

Pharmacologic Stress Testing
For patients unable to exercise adequately, pharmacologic stress testing may be appropriate. With SPECT MPI, appropriate patients can be converted from exercise to pharmacologic stress.6
Myocardial perfusion defects are typically graded by their extent and severity. In addition to qualitative evaluation of perfusion defects, semiquantitative interpretation of MPI helps standardize visual interpretation of scans, reduce the likelihood of overlooking significant defects, and provide an important semiquantitative index that is applicable to diagnostic and prognostic assessments.8
Current ASNC SPECT Imaging Guidelines recommend semiquantitative analysis using a segmental 5-point scale system on a 17-segment model of the left ventricle8:

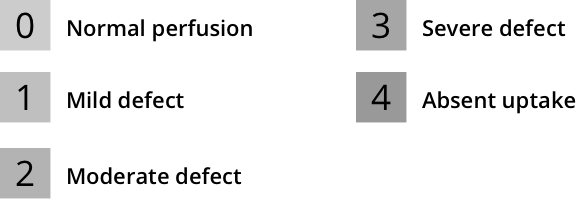
Left ventricular segmentation and scoring8,a
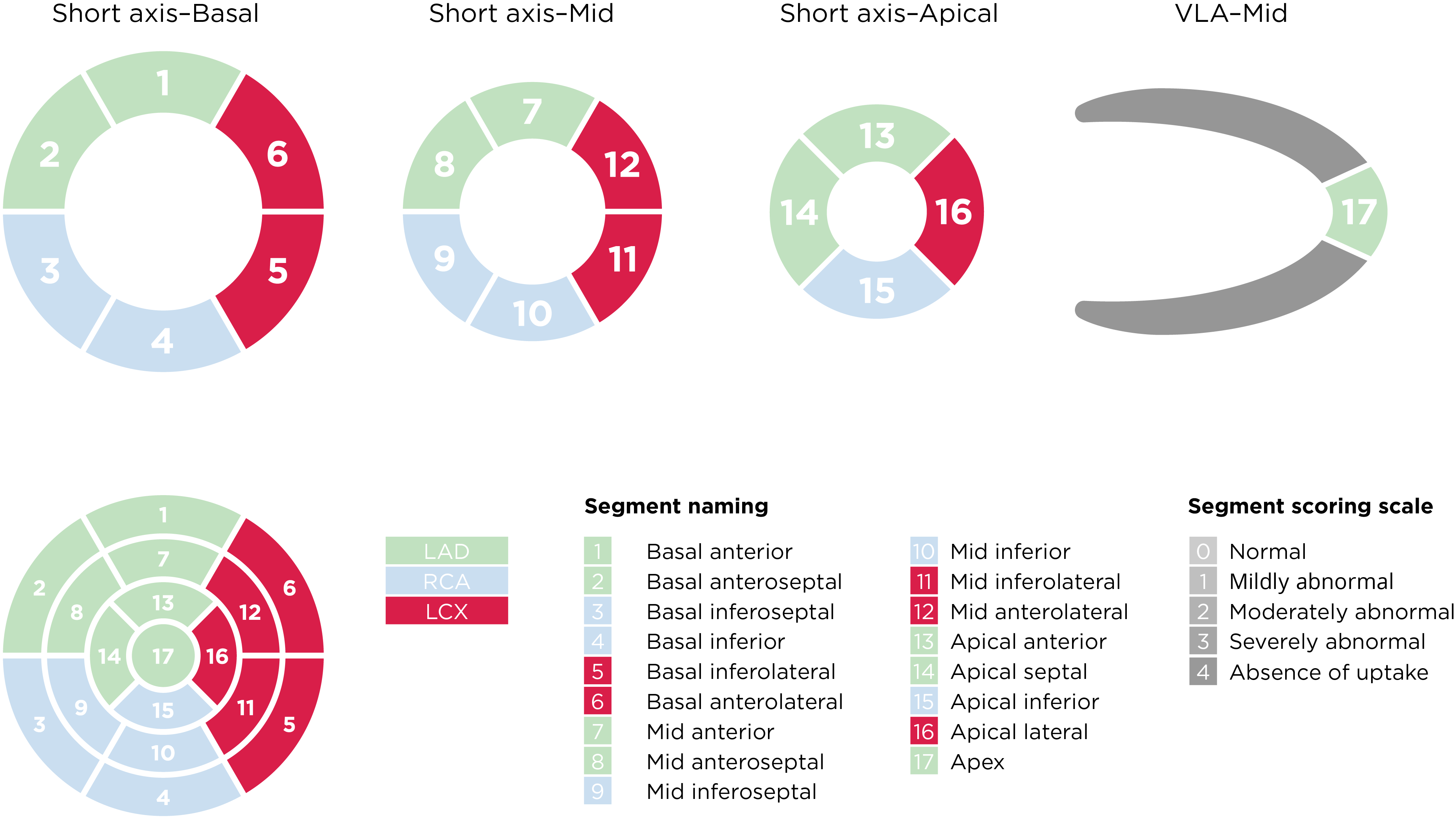
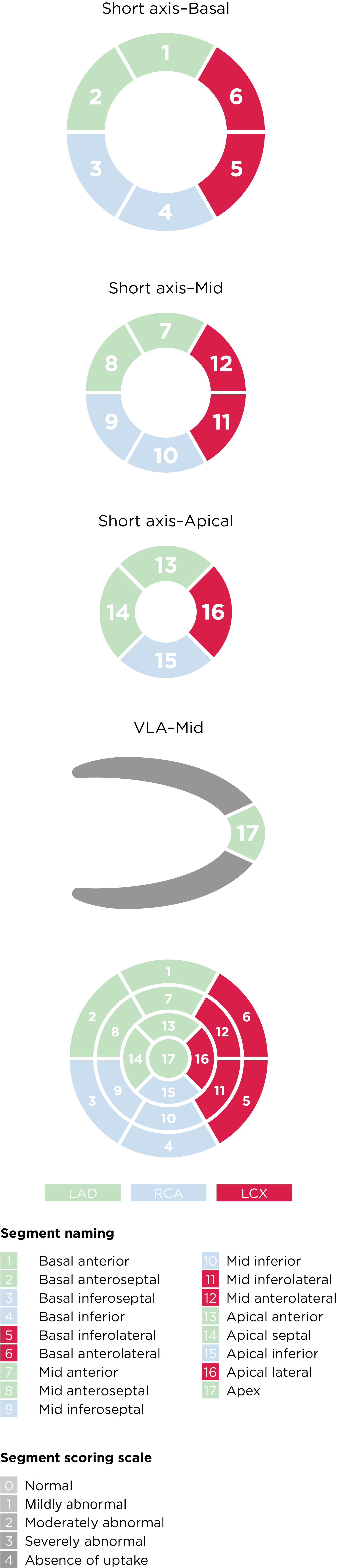
aAdapted from the American Society of Nuclear Cardiology; originally presented in Cerqueira MD, et al. J Nucl Cardiol 2002;9:240-5.
In addition to individual segmental scores, ASNC SPECT imaging guidelines recommend that summed scores be calculated. These scores represent the summed stress scores (SSS) of all segments and the summed rest scores (SRS) of all segments. The summed difference score (SDS) is the difference between the SSS and SRS8:
SDS is a measure of perfusion defect reversibility, reflecting inducible ischemia or hibernating myocardium.8
These semiquantitative metrics help guide decisions about the appropriate use of coronary revascularization.8
Functional assessment of wall motion and thickening as well as left ventricular ejection fraction (LVEF) also help stratify risk. According to ASNC guidelines, regional wall motion should be analyzed and classified using a semiquantitative scoring system8:

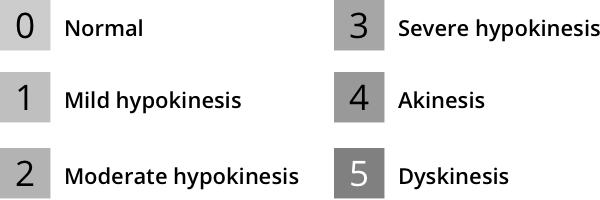
Software algorithms can help with semiquantitative scoring of wall motion and thickening.
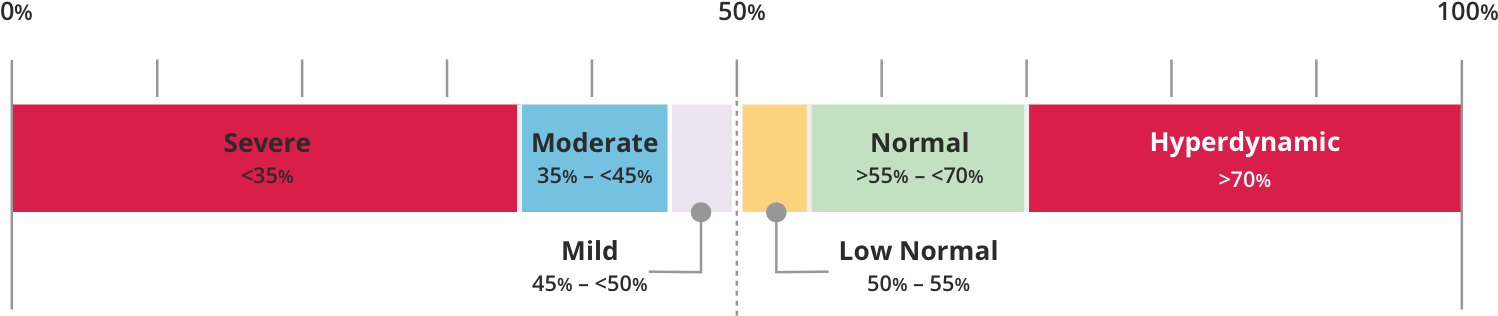
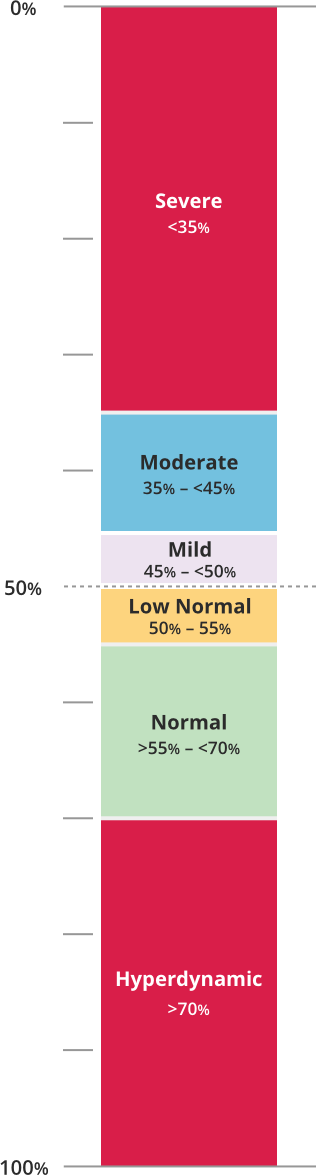
LVEF can be categorized as follows8:
References+
1. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. J Am Coll Cardiol 2012;60(24):e44-164. Erratum in: J Am Coll Cardiol 2014;63(15):1588-90. 2. Dorbala S, Di Carli MF. Nuclear cardiology. In: Libby P, Bonow RO, Mann DL, Tomaselli GF, Bhatt DL, Solomon SD, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 12th ed. Philadelphia, PA: Elsevier Inc, 2022. 3. Driessen RS, Raijmakers PG, Stuijfzand WJ, Knaapen P. Myocardial perfusion imaging with PET. Int J Cardiovasc Imaging 2017;33(7):1021-31. 4. Bateman TM, Heller GV, McGhie AI, et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: Comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol 2006;13(1):24-33. 5. Abbott BG, Case JA, Dorbala S, et al. Contemporary cardiac SPECT imaging—innovations and best practices: an information statement from the American Society of Nuclear Cardiology. J Nucl Cardiol 2018;25(5):1847-60. 6. Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: stress, protocols, and tracers. J Nucl Cardiol 2016;23(3):606-39. Erratum in: J Nucl Cardiol 2016;23(3):640-2. 7. DePuey EG, Mahmarian JJ, Miller TD, et al. Patient-centered imaging. J Nucl Cardiol 2012;19(2):185-215. Erratum in: J Nucl Cardiol 2012;19(3):633. 8. Dorbala S, Ananthasubramaniam K, Armstrong IS, et al. Single photon emission computed tomography (SPECT) myocardial perfusion imaging guidelines: instrumentation, acquisition, processing, and interpretation. J Nucl Cardiol 2018;25(5):1784-846.